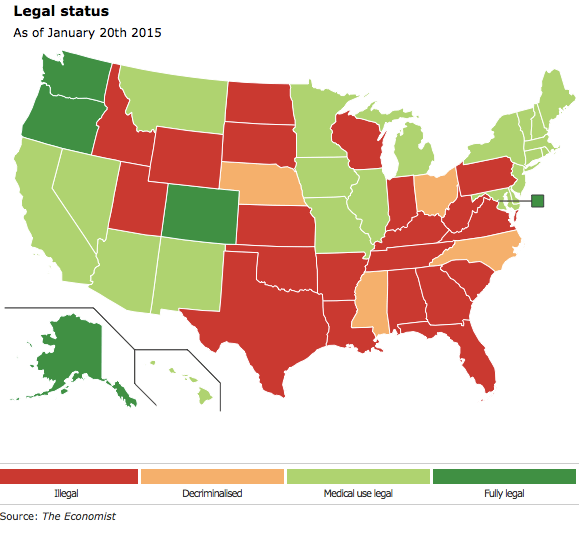
It’s been a long and rough road for a push toward…
universal legalization of marijuana in the US. Since the early beginnings in California many decades ago with medical marijuana to the latest trail being blazed from states like Colorado, Oregon, Washington, and Alaska, this has certainly become a growth driven marketplace full of opportunity. It seems like those entering the space are facing the “kid in a candy store” mentality as so many industry assets can be applied to this new sector. From consulting services to biotech, there is a myriad of options for companies to focus on during these early stages. But the question remains, “Are ‘pot’ stocks really delivering for investors?” So, which stocks should you be in right now?
After facing heavy consolidation in the market during the late summer months, I believe the investment community started to educate themselves on the dangers of investing on hype and though there are still day traders out there that continue to “chase on speculation”, the average investor is not so average anymore, especially after a chunk of those would be marijuana companies saw their stocks get halted last year. During January and now into February, the buzz is starting to grow yet again and it’s been the cannabis-focused companies who’ve once again captured the public’s attention.
Oxis International (OXIS)
Oxis International, Inc. operates through its subsidiary Oxis Biotech, Inc. The company has recently come into the cannabis biotech space and may represent the greatest potential reward of all the companies on this list considering where the company’s valuation stands today, an approximate market cap of just $15million.
Over the last 45 days, Oxis shares have seen highs of $0.039 and over recent weeks, the company has made several key announcements that put this on the list of top potential cannabis investments in 2015. Not only has Oxis executed definitive agreements licensing certain assets for the treatment
of multiple myeloma but the company has also brought on some of the foremost authorities in research, especially those focused on cannabinoid research. Starting with Dr. Sean Xie (Dr. Xiangqun Xie), his research work is beyond the proof-of-principle stage and has already shown to successfully identify several CB2 ligands with “nanomolar receptor binding affinity” (US patents). Currently, he has established collaboration with MD experts at the University of Pittsburgh Medical Center for in vivo animal evaluation.
Furthermore, the Company also announced that Dr. James J. Mulé, Ph.D and Dr. Lisa A. Haile have joined the Scientific Advisory Board. Dr. Mulé is a Special Government Employee of the NCI and the FDA. There is literally no better person to have on your team as a biotech company than someone who works for the FDA that can oversee and manage the different trial phases for FDA drug approval.
Among many of his accomplishments, Dr. Mulé is recognized for his translational research studies in cancer immunotherapy. Mulé previously worked with Oxis CEO Tony Cataldo at Mr. Cataldo’s last biotech company, Genesis Biopharma (previous GNBP), which later became Lion Biotechnologies (LBIO). Under the helm of Mr. Cataldo, Genesis grew from a penny stock with a mere $2million market cap to a $250million market cap now trading around $8 a share. Needless to say, early investors were beyond thrilled.
I like to invest in management with a proven track record. People that have been through the gauntlet and came out on top because they are more likely to do it again than a newbie is to make it big for the first time. In addition, CEO Tony Cataldo has been quoted stating that he believes their patents and data are comparable to cannabis biotech juggernauts GW Pharma and Insys Therapeutics, two companies also on this Top 5 list who both sport market caps around $1.5Billion. But moving on.
Dr. Haile, Ph.D. currently serves as Co-Chair, Global Life Sciences Sector at DLA Piper and has substantial experience when it comes to FDA counseling and licensing strategy. She is also noted as being one of the nation’s best lawyers when it comes to successfully bringing innovation to market and making sure it remains proprietary to the company. According to her biography, Dr. Haile has extensive experience with US and International patent preparation and prosecution relating to novel genetically altered organisms, including plants, antisense molecules, peptides, proteins, DNA, antibodies, vaccines, diagnostics and therapeutics, as well as patentability, infringement and validity determinations, licensing and strategic counseling. This should add another layer of professional advisory to the Oxis Scientific Board especially with the current path the company has begun to head down in consideration of the multiple myeloma licensing.
As far as paid in capital is concerned, Oxis shows something more similar to a large cap company and reports slightly over $82M in additional paid in capital. Therefore, it could be very possible that the CEO who took Genesis from a micro-cap stock to where it sits today as Lion Biotech, may be gearing up to do it all over again. A strong team to conduct R&D is crucial to future success. Additional paid in capital is very important when it comes to making investment decisions in many biotechs generally because most of these are pre-revenue through the multiple stages of R&D and through the different phases of FDA approval. This valuation method has been used because when you’re not selling product, how can success be determined? By utilizing “additional paid-in-capital” and various patent valuations along with other financial reporting documentation.
GW Pharmaceuticals (GWPH)
Market leader GW Pharmaceuticals (GWPH) started to come back from a sell-off period in late December. Anticipator buying prior to GW’s Sativex results saw the stock rise from $67 to highs of $80.68. Unfortunately for the company and the stock as of recent weeks, results were dismal. According to coverage from 24/7 Wall St., “There was little difference in the adverse event pattern between Sativex and a placebo. There were 38, or 19%, withdrawals due to adverse events on Sativex, compared to 29, or 15%, on the placebo.”
GW’s stock price has suffered due to this event and has seen a decline by as much as 10% from early January highs. There may still be opportunity here as GW has stated that it is confident in the ability for Sativex to relieve cancer. Justin Gover, GW’s CEO, explained, “We have two additional pivotal Phase 3 trials ongoing which, if positive, would still allow us to submit a New Drug Application with the US FDA. We look forward to results from these two further studies later this year.”
Furthermore, something may have also been overlooked on the day that GW released its Sativex results and that is the update on its other drug and patent filing progress. GW announced an update on its development program for Epidiolex(R) focusing on Phase2/3 trials commencing during the first quarter of the year with high expectations for these trials. This is where the opportunity lies with GW Pharma. A sell-off from bad results for one drug has investors blind to the great news behind another. Additionally, and what may be of added interest as the cannabis space leans more toward a biotech focus, is the company’s patent filing which protects the use of CBD in the treatment of partial seizures.
The U.S. Patent and Trademark Office has issued a Notice of Allowance for U.S. Application Serial Number 13/380,305. This is usually one of the last steps before a patent is actually issued as a Notice of Allowance is issued after the USPTO makes a determination that a patent can be granted from an application. To the benefit of GW, an issued patent from this application will provide an exclusivity period until June 2030. This is important from the fact that many companies are pushing for therapies dealing with epilepsy among other things and if/when GWPH’s patent is approved, they will have a large corner within that market in the near term.
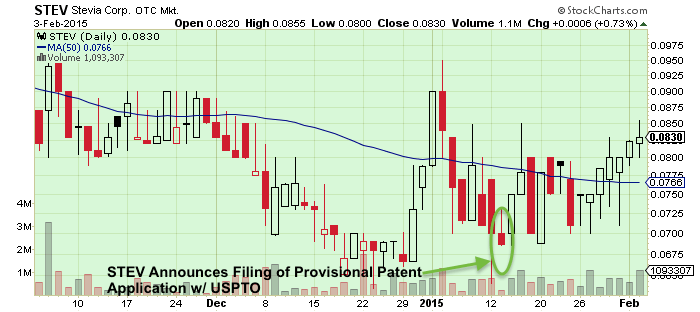
Stevia Corporation (STEV)
Also looking to benefit from patent filing is Stevia Corporation (STEV). The company, which recently switched focus from sugar alternatives to cannabis, has released a series of press relating to the filing of provisional patents for its CBD based pain medication, which is actually a mix of cannabidiol and ibuprofen. In a January 29th release, George Blankenbaker, President of Stevia Corp stated, “We believe that there will be significant advantages of using these four very effective drugs in combination with cannabidiol as opposed to using these drugs alone. Acetaminophen, Ibuprofen, Naproxen and Aspirin generate billions of dollars in sales each year. Our four provisional patent applications include 63 claims in total and we look forward to filing the utility patents over the next 12 months. We strongly believe that our intellectual property strategy, if successfully implemented, will provide us a competitive advantage over other healthcare companies using cannabinoids for human healthcare.”
The obvious advantages should these patents be approved are similar to that of GW: offering a larger advantage to being the “first to market” organization and to further limit other companies from throwing a hat into the ring. Risks abound however especially with the current state of Stevia’s market. The most recently filings show that that company obtains most of its money from farming activities. Even though the quarterly shows a cost of revenue of nearly $2m, its additional paid in capital amounts to $16.3million. Should this patent process not pan out, there is still an entirely established and revenue producing side of Stevia that won’t depend on cannabis. On a market side, Stevia’s stock has begun to recover since hitting lows earlier in January of $0.064. As of this past week, STEV has seen a rebound of roughly 30% since releasing announcements on this latest patent filing.
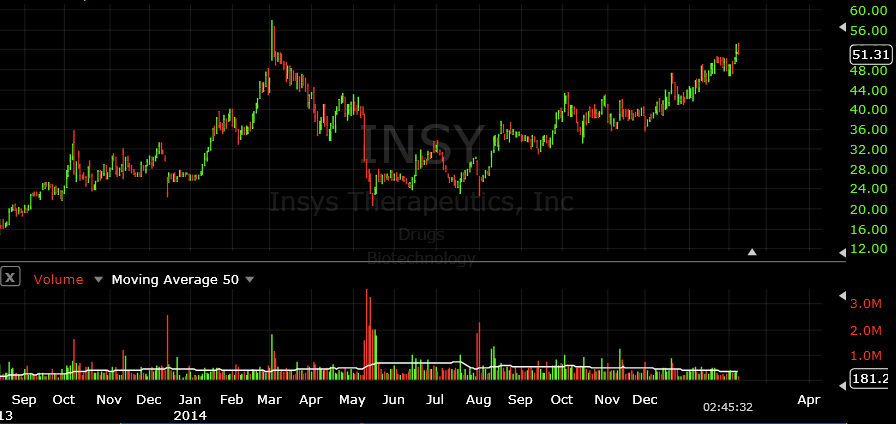
Insys Therapeutics (INSY)
Insys Therapeurtics (INSY) is a company who’s stock could have been invested in early last year and then completely forgotten about. Unlike many of the marijuana stocks back in early 2014, INSY didn’t have any type of short-term parabolic move but has remained on a consistent uptrend over the last year. With $205M in additional paid in capital, $47M in cash & equivalents, as well as net revs of nearly $200M for 9months ended Sept 30 2014, Insys is one of the better performers when it comes to a company dealing in the cannabis marketplace. As noted in the last financial report, the company’s drug, Subsys, accounted for almost 100% of its 2014 year-to-date revenue with its gross margin reporting in at approximately 91%. These results should be favorable to investors and a reason to consider INSY.
Over recent weeks, the stock price has continued to rise. In fact, since the initial report on Cannabis companies was released, Insys shares have already jumped in price by as much as 13% as the stock approaches highs it hasn’t seen since early 2014. On January 26, 2015 the company announced that the U.S. Food and Drug Administration granted orphan drug designation (ODD) to its Liposomal Encapsulated Paclitaxel (LEP) candidate for the treatment of ovarian cancer. Similar to Oxis International, Insys focuses on synthetic versions of CBD as compared to other cannabis biotech plays that look at the organic versions of the drug. The main difference is how the “medicine” is derived and converted into an effective therapy. Some companies find that the synthetic approach offers a more consistent product as compared to organic methods, which may be a reason that Insys has been able to bring its products to market so easily.
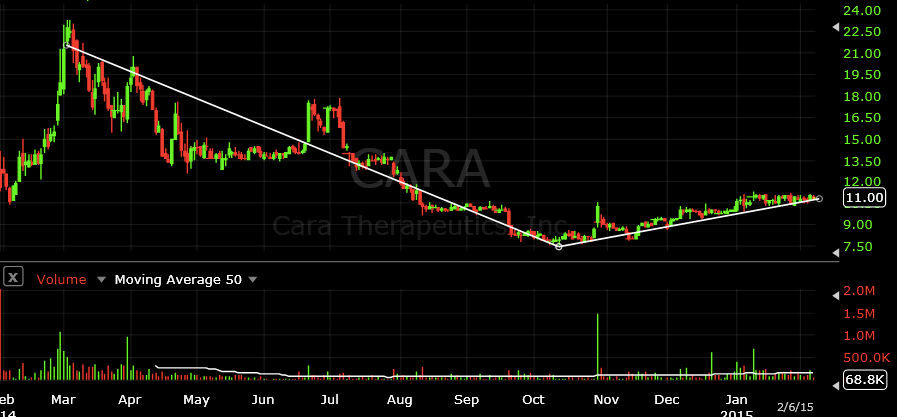
Cara Therapeutics, Inc. (CARA)
On a clinical stage, Cara Therapeutics has been gaining ground since the third quarter of 2014. The company focuses pain therapies and utilizes kappa opioid receptors to accomplish this. Unlike Insys who uses a sublingual spray, Cara employs the use of intravenous (IV) means. Its CR845 candidate has demonstrated significant pain relief as well as a favorable safety and tolerability profile in three Phase 2 clinical trials in patients with acute postoperative pain. According the Cara, top-line results from an initial Human Abuse Liability trial indicate that I.V. CR845 met the trial’s primary endpoint by demonstrating “highly statistically significant lower “drug liking” scores as measured by visual analog scale (VAS) Emax (p <0.0001) when compared to I.V. pentazocine, a Schedule IV opioid receptor agonist.”
Aside from clinical trials in the US, Cara’s product is already licensed to Maruishi Pharmaceutical Co., Ltd. in Japan and Chong Kun Dang Pharmaceutical Corporation in South Korea. However, these agreements have not generated significant revenues and are where the risk resides with this company (outside of the universal market risk all stocks hold). After looking deeper into the filings, it would appear that Cara’s only clinical stage drug is its CR845 and should this fail, so may the company.
Despite these factors, the company still shows cash and cash equivalents of nearly $60M and an additional paid in capital of over $130M. With roughly $11m use in operating activities, Cara should be able to sustain operations for the near term and that’s even assuming the company does nothing else to generate other revenues from licensing. Furthermore, since mid-October of 2014, the stock price has been on a consistent uptrend and has moved up by as much as 49.5% from lows of $7.53 to highs of $11.26 earlier this year. This also marks the first time since Colorado legalized recreational marijuana that Cara shares have seen any kind of consistent uptrend in stock price for any sustained period of time longer than a few weeks.
Important Stats After Legalization of Marijuana Turns 1
- Colorado’s annual demand for marijuana is 130.3 metric tons, which is equal to 36.8 million “eighths” of cannabis flower.
- 23% of Colorado’s user population consumes cannabis almost every day.
- 90% of recreational pot sales are attributed to out-of-state tourists at shops in mountain resort communities.
- Seven percent of Colorado’s annual pot demand is purchased by out-of-state tourists.
- Recreational marijuana sales, as counted from January to October topped out at $246,810,599.03
- Colorado reported total medical marijuana sales from January to October of $326,716,273.59.
- Colorado has brought in $60.1 million via taxes, licenses and fees on recreational and medical marijuana, from January to October.
Conclusion
Last year was definitely a learning year for many. From January to May, there was a lot of money that could have been made and lost but despite these shortcomings as this new industry emerged, investors have become more educated through the trials that this market has demonstrated. There are so many options for investment from consulting services to biotech and for investors, the navigation of this niche may seem a bit unclear. I, for one, will continue to lean on management and financials to support my own investment decisions and these are just a few which have passed the initial test for potential top performers this year.
MAPH Enterprises, LLC | (305) 414-0128 | 1501 Venera Ave, Coral Gables, FL 33146 | new@marijuanastocks.com











4 comments
Stevia Corp. Investor Conference Call – January 29, 2015 – Listen to it on YouTube https://www.youtube.com/watch?v=joYVv0QUomw
If only the clowns had any idea how much is consumed by old baby boomers!
If only they had a clue of how much is grown without ever being noticed!
To the victor go the spoils
This should apply to the Marijuana revolution as well.
We have them down and by the throat. Let’s finish ’em off NOW.
No licenses should go to people that weren’t involved in this victory.
Only those who lost jobs or were incarcerated etc should get our support $$$ Period. We are a cohesive group and if we boycott those pretenders shops, they will soon be out of business.
I read about one investor that owned over 60 licenses. Boo and boycott.
Those $30,000 licenses will be worthless real fast.